
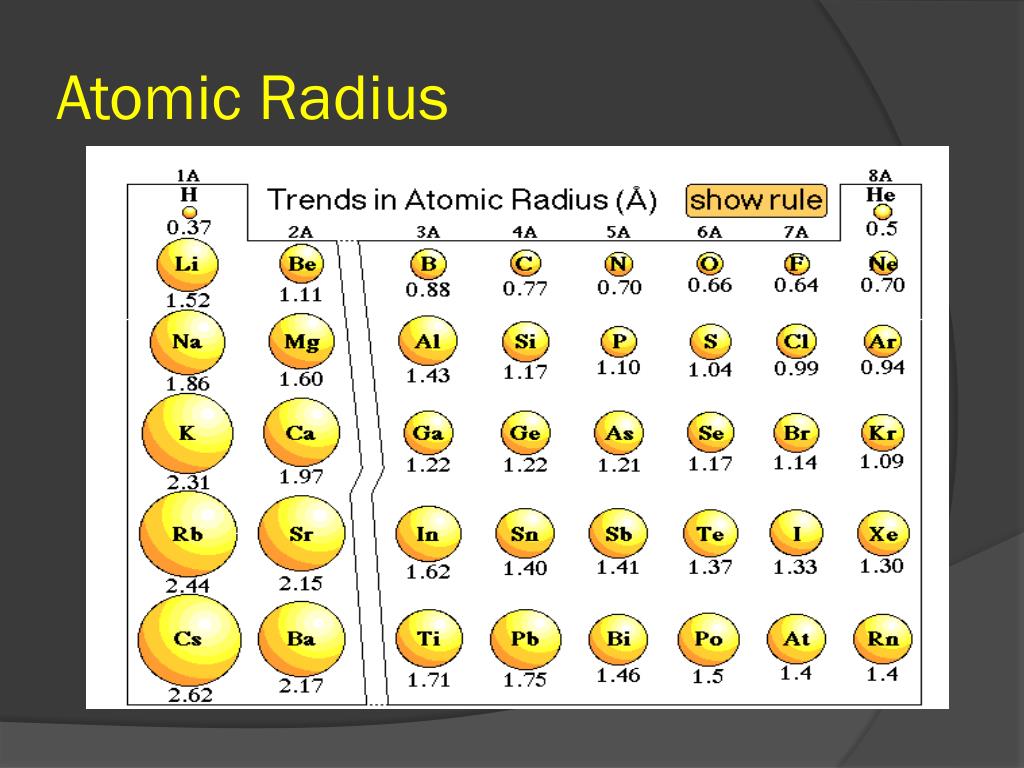
From top to bottom in a group, orbitals corresponding to higher. Thus, helium is the smallest element, and francium is the largest. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. That is how the relation was derived from simple geometry. Atomic radii vary in a predictable way across the periodic table. double explosion-affected people), nine of whom claimed to be in the blast zone in both cities.
The Doubly Atomic Bombed of Hiroshima and Nagasaki documented 165 nij hibakusha (lit. Oxygen Data Oxygen Atomic Radius State at 20 C Gas Uses Used in steel making, welding, and supporting life. The radius of total destruction was about 1.6 km (1 mi), followed by fires across the northern portion of the city to 3.2 km. In the case of Oxygen There are cool facts about Oxygen that most don't know about. Ok, so what is the atomic radius of an atom of O Note: Learn more about the atomic radius here. For comparison, the radius of a typical bacterium is 1 × 10-6 m and the radius of a human hair is about 1 × 10-4 m. Therefore as an ionic solid it has octahedral holes in the middle of the larger #O^-1)R# is an approximation arrived at by assuming that all of the atoms in the ionic lattice are closest-packed hard spheres touching each other. All atoms have a (theoretical) atomic radius, even Oxygen.

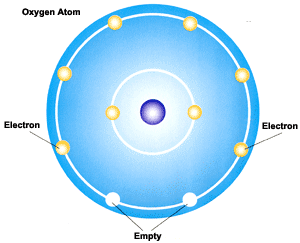
Looking at the picture, MgO seems to have the lattice structure of NaCl. Up to date, curated data provided by Mathematica 's ElementData function from Wolfram Research, Inc.


 0 kommentar(er)
0 kommentar(er)
